Sample problems and solutions in Chemistry
Chemistry : Equilibrium constants, Enthalpy, Gibbs free energy, Arrhenius equation, rate constant, rate of reaction
Problem 1: Consider a gas phase reaction involving the synthesis of ammonia from hydrogen and nitrogen at 350 oC
Starting with 1 mole of and 3 moles of
, and no
to start with, Find
- An expression for the equilibrium constant
in terms of the equilibrium conversion x
- If the equilibrium conversion at 298K and 1 atm is 0.6, find the equilibrium constant
for the above reaction at 298K and 1 atm.
Solution: = 16.33 Please contact via email for a detailed solution
Problem 2: For the gas phase reaction involving reactants X and Y, the individual heat capacities are 3 and 4 kcal/mol/K respectively while that of the products R and S are 6 and 7 kcal/mol/K respectively. Reactants X and Y react according to reaction below to form products P and Q at 227 oC and 5 atm . Find the heat of the reaction at 327 oC if the heat of the reaction at 227 oC is 100 kcal/mol.
Solution: = 3700 kcal/mol. Please contact via email for a detailed solution
Problem 3: Isomerization of Catechol using hydrogen peroxide produces an equilibrium mixture of three isomers Catechol (ortho), Resorcinol (meta) and Hydroquinone (para) as represented by the scheme below
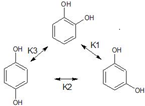
If the initial concentration of pure catechol is Co , find the concentrations of the three isomers at equilibrium in terms of the given constants. Make suitable assumptions if required.
Solution: Please contact via email for a detailed solution
Problem 4: Pyrolysis (thermal cracking) of large chain hydrocarbon n-decane into smaller hydrocarbons at 627 oC was found to have an activation energy of 32.86 kJ/mol and reaction rate r . If the same reaction was carried out at 927 oC, the reaction rate was found to be mr . Find m to the closest positive integer possible.
Solution: m = 3 Please contact via email for a detailed solution
Practice Papers for free download
Chemistry Set 1
Chemistry Set 2
Chemistry Set 3
Chemistry Practice paper 1
Chemistry Practice Paper 2
Chemistry Practice paper 3
Chemistry Practice paper 4
Chemistry Practice Paper 5
Chemistry Practice Paper 6